What Is 21 CFR 820?
The Code of Federal Regulations (CFR) and Title 21 (known as 21 CFR) is a set of regulations set by the Food and Drug Administration (FDA) for everything related to food and drugs. 21 CFR 820 provides the requirements for good manufacturing practices that manufacturers must follow for all stages of development. It governs the methods, facilities, and controls used for medical device design, manufacturing, packaging, labeling, storage, installation, and service. This is important for manufacturers to ensure they are producing medical devices that are effective and assure patient safety.
MATLAB® and Simulink® enable organizations to comply with 21 CFR 820 requirements for software aspects of a medical device by complying with the software life cycle process requirements of IEC 62304. MATLAB and Simulink provide a more efficient approach to developing high-integrity software for medical devices by leveraging computational Model-Based Design. At the center of this methodology is a system model that spans requirements development, architectural analysis and specification, detailed design, implementation, verification, and validation. You refine your design through simulation and rapid prototyping, then generate code to implement applications in embedded devices, apps in cell phones, and/or enterprise applications. MATLAB and Simulink products include tools for requirement traceability, modeling and simulation, testing, and verification for implementing Model-Based Design, which ensures compliance with IEC 62304 and 21 CFR 820. These tools and workflows are deemed suitably validated for all software safety classes of IEC 62304 by TÜV SÜD.
21 CFR 820 Subpart C—Design Controls addresses the aspects of device development where Model-Based Design can be an effective set of tools and processes.
Design inputs can be analyzed with computational modeling and simulation (CM&S) to help validate that they meet market requirements like the needs of the user and patient. Requirements analysis with computational models can be an effective way to determine the consistency and completeness of these requirements.
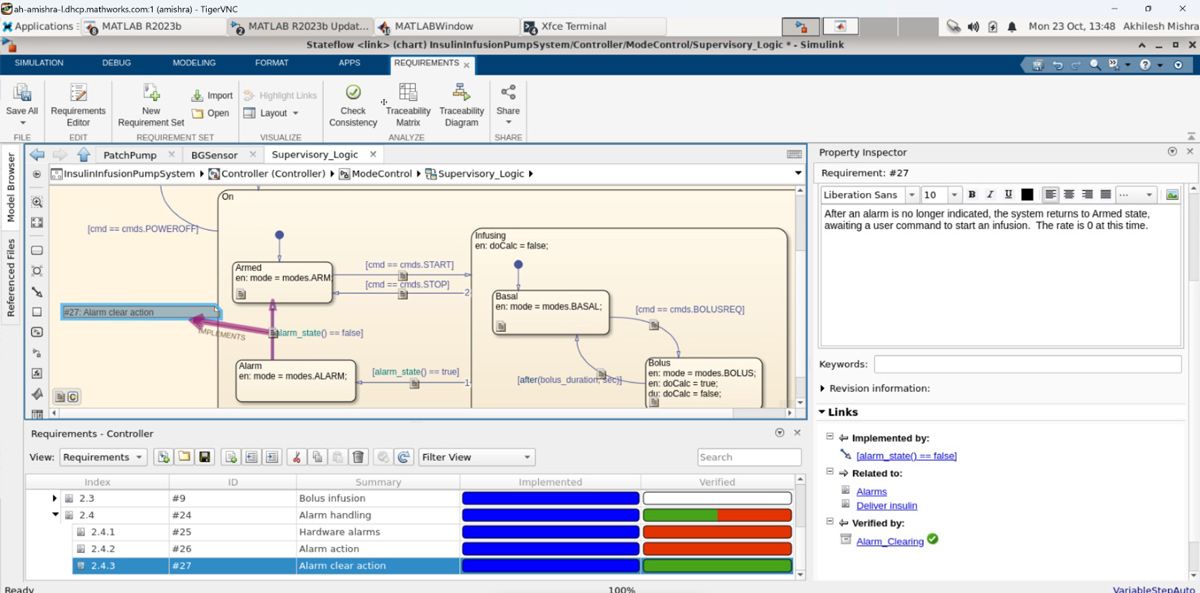
Using Requirements Toolbox™, the user can implement bidirectional traceability between the requirements and computational model to determine the consistency and completeness of the requirements. Learn how you can use a Model-Based Design workflow with Requirements Toolbox to develop an insulin infusion pump design—from systems engineering to testing.
Design outputs can be verified at each stage of the software development workflow, demonstrating compliance with requirements via traceability analysis and simulation test scenarios. The design verification features of MATLAB and Simulink tools and workflows can automate the execution of test suites to provide evidence of process adherence.
Design transfer from design-intent models to source code implementation can be automated via production code generation, leaving software integration work and integration verification as the remaining software development lifecycle tasks.
Models can be partitioned to reduce impact during design changes, and the dependencies of the models are documented and traceable to help perform impact analysis and reveal and justify the necessary level of reverification and revalidation efforts.
MATLAB and Simulink generate reports that can be used to gather model structure and details, traceability, and test and verification results for documentation of your achievements into your design history file.
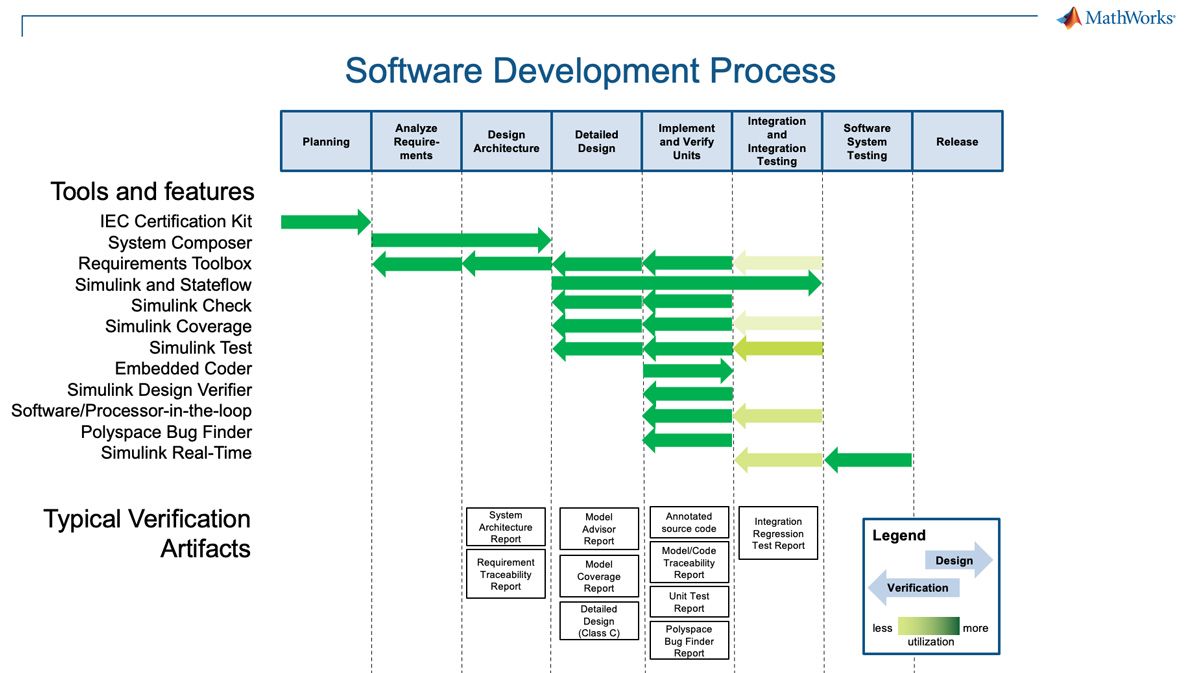
Software development process details are implied within a subset of the high-level design controls and described in supplemental documents such as the FDA’s General Principles of Software Validation guidance document. Another example is IEC 62304, which is an agreed-on international standard that addresses software lifecycle process requirements for medical device software. MATLAB and Simulink are compatible with these detailed requirements and can provide evidence of adherence. For a summary view, see the diagram below, which shows some common usage scenarios of the tools and typical documentation artifacts that they can produce. To summarize, MATLAB and Simulink provide many capabilities to accelerate the 21 CFR 820 Subpart C—Design Controls regulations for medical device development by providing an integrated solution to enable:
- Design input validation
- Design output verification
- Design transfer automation
- Design changes impact analysis
- Document generation for design history files
To learn about using modeling and simulation for medical device development, please see Best Practices for IEC 62304 compliance with Model-Based Design.